FDA approves first rapid Covid-19 test by Cepheid, YML design partner
By YML
YML partnered with Cepheid to create the interface.
Throughout the Covid-19 pandemic, a major problem has been access to reliable, quick testing. Cepheid, a Silicon Valley-based molecular diagnostics company and partner to YML, created a breakthrough test that dramatically cuts down Covid-19 detection time from three to four days to just 45 minutes. The U.S. Food and Drug Administration approved Cepheid's first rapid detection on March 21st.
The GeneXpert Xpress, a product created by Cepheid, is the first rapid coronavirus diagnostic test.


The U.S. FDA has been pushing to expand screening capacity for the virus while the World Health Organization has called for "order and discipline" in the market for health equipment needed to fight the outbreak.
Cepheid received an emergency use authorization from the FDA for the test, which will be used primarily in hospitals and emergency rooms. The company plans to begin shipping it to hospitals this week.
YML has been a design partner to Cepheid since December 2019, tasked with designing the product’s user interface to enable as much as speed and clarity within the test process as possible.
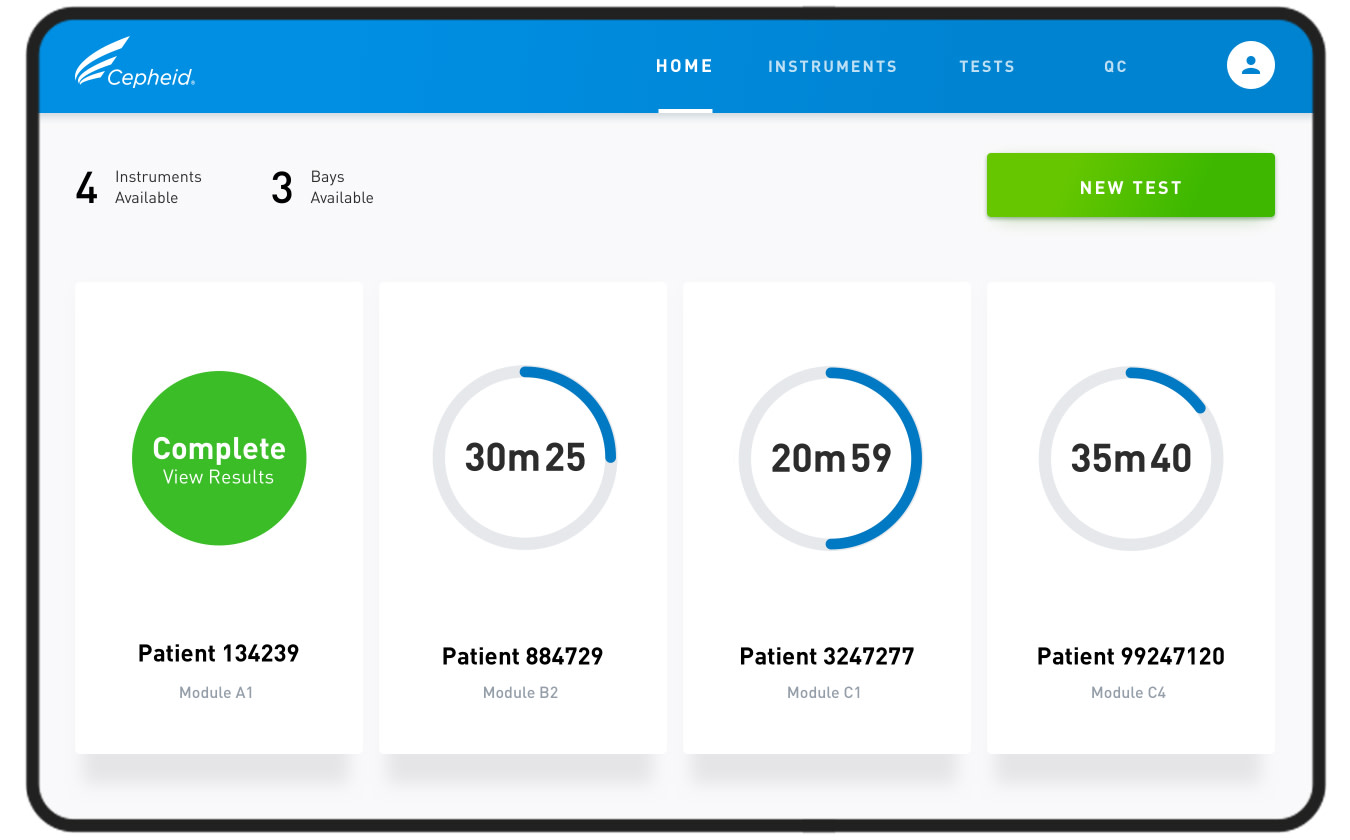

Cepheid's test functions through the GeneXpert Xpress (above), an instrument designed for mass accessibility. By inputting a single test in each of its four cartridges, the GeneXpert can run up to 16 tests simultaneously, depending on how many instruments are connected. YML's team was responsible for reimagining the user experience on the built-in, attached touch screen, which is a landscape tablet running on Windows.
With 23,000 stations worldwide, Cepheid is making testing more accessible everywhere. Based on its series of products, including the GeneXpert Xpress and others, Cepheid has the capacity to run 80 tests simultaneously, which means 2500 tests a day if it’s running for 24 hours.
“We have developed a test that provides reference lab-quality results in multiple settings where actionable treatment information is needed quickly.”
Dr. David Persing, MD, PHD Chief Medical and Technology Officer Cepheid
Cepheid's collaboration with YML included internal and external clients and constant iteration to create a final, impactful product with the potential reach millions of people all over the world, and most importantly, flatten the curve.
YML's design collaboration with Cepheid is ongoing.
Reach out to YML with any questions about the work or collaboration.